Difference between revisions of "Ilmenite Reduction"
m (dummy edit to remove vandalism) |
|||
| Line 1: | Line 1: | ||
== Introduction == | == Introduction == | ||
| − | [[Reduction|Reducing]] [[ilmenite]] (FeTiO<SUB>3</SUB>) to produce [[oxygen]], [[iron]], and [[titanium]] in a lunar context has produced a number of proposals, many of them specifically aimed at oxygen production. Ilmenite is attractive for this purpose as the iron oxides it contains require less energy to reduce than any other oxide on the lunar surface. For this reason, proposals which have oxygen production as the primary goal usually focus on reduction of the iron content of ilmenite. | + | [[Reduction|Reducing]] [[ilmenite]] (FeTiO<SUB>3</SUB>) to produce [[oxygen]], [[iron]], and [[titanium]] in a lunar context has produced a number of proposals, many of them specifically aimed at oxygen production. Ilmenite is attractive for this purpose as the iron oxides it contains require less energy to reduce than any other oxide on the lunar surface. For this reason, proposals which have oxygen production as the primary goal usually focus on reduction of the iron content of ilmenite. |
| − | This is | + | ==Hydrogen Reduction== |
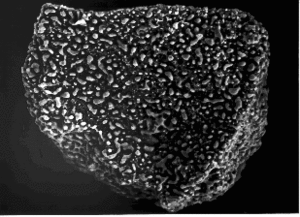
| + | [[image:ilmen_reduced.GIF|thumb|Lunar Ilmenite reduced at 1050°C by hydrogen for 3 hrs]] | ||
| + | [[Hydrogen]] reduction is one method currently being tested by many Universities. Products of hydrogen reduction are free [[iron]], [[rutile|titanium dioxide (TiO<sub>2</sub>)]], and [[water]], which is [[Water Splitting|split]] to recover the hydrogen and produce [[oxygen]]. | ||
| + | |||
| + | The basic process is to separate ilmenite from lunar soil, crush it to a fine powder to maximize the surface area, and then heat it in an enclosed reaction vessel in the presence of hydrogen gas. The steam produced in the reaction is then condensed and [[Water Splitting|split]] to produce oxygen and recover the hydrogen. | ||
| + | |||
| + | This process is best utilized if the plant is sited in a location in which ilmenite composes a high fraction of the soil. | ||
| + | |||
| + | The reaction sequence is: | ||
| + | *''Reduction'': FeTiO<SUB>3</SUB>+H<SUB>2</SUB> ---->Fe+TiO<SUB>2</SUB>+H<SUB>2</SUB>O | ||
| + | *''Water Splitting'': 2H<SUB>2</SUB>O ---->2 H<SUB>2</SUB>+ O<SUB>2</SUB> | ||
| + | *''Net Reaction'': 2FeTiO<SUB>3</SUB>----> 2Fe+2TiO<SUB>2</SUB>+ O<SUB>2</SUB> | ||
| + | |||
| + | The iron produced in the process could be separated out by [[Carbonyl process| carbonyl extraction]], or by grinding the result again and using a magnet. The [[rutile|titanium dioxide]] could also be further [[Lunar Titanium Production|reduced to produce metallic titanium and additional oxygen]]. | ||
==Carbothermal Reduction== | ==Carbothermal Reduction== | ||
| Line 17: | Line 30: | ||
<BR>Where n represents the number of TiO<sub>2</sub> molecules | <BR>Where n represents the number of TiO<sub>2</sub> molecules | ||
| − | + | ===Reduction with CO=== | |
| + | This reaction is based on a fluidized bed scheme which is similar to large scale proposals for Hydrogen Reduction. The product of CO reduction of Ilmenite is [[Carbon Dioxide|Carbon Dioxide (CO<sub>2</sub>)]], which is [[Lunar Carbon Production#Direct CO2 Electrolysis|reduced]] to [[Carbon Monoxide|CO]] and [[oxygen]]. The [[Carbon Monoxide|CO]] is recirculated, and the oxygen stored.<ref>http://www.uapress.arizona.edu/onlinebks/ResourcesNearEarthSpace/resources04.pdf page 9</ref>. The presence of solar wind implanted carbon in the regolith will allow the recovery of additional carbon if recycling efficiency is sufficiently great, though a method for dealing with evolved methane (CH<sub>4</sub>, from hydrogen present in the regolith) would be required. The CO reduction of ilmenite is slower than the H<sub>2</sub> process, but by less than an order of magnitude for any given temperature. | ||
| + | |||
| + | The reaction sequence is: | ||
| + | <BR/><BR/> | ||
| + | ''Reduction'':<BR/> | ||
| + | FeTiO<sub>3</sub> + CO ---->Fe + TiO<sub>2</sub> + CO<sub>2</sub> | ||
| + | <BR/> | ||
| + | ''Endothermic cracking'':<BR/> | ||
| + | 2CO<sub>2</sub> ----> 2CO + O<sub>2</sub> | ||
| + | <BR/> | ||
| + | ''Net Reaction'': <BR/> | ||
| + | 2FeTiO<sub>3</sub> + 2CO ---->2Fe + 2TiO<sub>2</sub> + 2CO + O<sub>2</sub> | ||
| + | <BR/> | ||
==Methane Reduction== | ==Methane Reduction== | ||
| Line 31: | Line 57: | ||
| − | + | == References == | |
| + | <references/> | ||
== Related Pages == | == Related Pages == | ||
Revision as of 15:32, 29 October 2011
Contents
Introduction
Reducing ilmenite (FeTiO3) to produce oxygen, iron, and titanium in a lunar context has produced a number of proposals, many of them specifically aimed at oxygen production. Ilmenite is attractive for this purpose as the iron oxides it contains require less energy to reduce than any other oxide on the lunar surface. For this reason, proposals which have oxygen production as the primary goal usually focus on reduction of the iron content of ilmenite.
Hydrogen Reduction
Hydrogen reduction is one method currently being tested by many Universities. Products of hydrogen reduction are free iron, titanium dioxide (TiO2), and water, which is split to recover the hydrogen and produce oxygen.
The basic process is to separate ilmenite from lunar soil, crush it to a fine powder to maximize the surface area, and then heat it in an enclosed reaction vessel in the presence of hydrogen gas. The steam produced in the reaction is then condensed and split to produce oxygen and recover the hydrogen.
This process is best utilized if the plant is sited in a location in which ilmenite composes a high fraction of the soil.
The reaction sequence is:
- Reduction: FeTiO3+H2 ---->Fe+TiO2+H2O
- Water Splitting: 2H2O ---->2 H2+ O2
- Net Reaction: 2FeTiO3----> 2Fe+2TiO2+ O2
The iron produced in the process could be separated out by carbonyl extraction, or by grinding the result again and using a magnet. The titanium dioxide could also be further reduced to produce metallic titanium and additional oxygen.
Carbothermal Reduction
Oxygen can be retrieved from Ilmenite (FeTiO3) and Rutile (TiO2) by means of carbothermal reduction. In experiments, powdered carbon and powdered ilmenite/rutile were evenly mixed and then heated to 1500 degrees Celsius. The end products of this reaction are Oxygen and a high strength Ceramic-metal composite (Cermet) of Iron (Fe) and Titanium Carbide (TiC) which has high chemical stability. The amount of reinforcing TiC ceramic in the matrix can be controlled via the amount of rutile and carbon used[1]. While this method provides a means of retrieving all of the oxygen from ilmenite/rutile and a potential for producing reinforced, high performance and wear components and cutting tools from lunar regolith, it is at the cost of highly valuable carbon needed for biological processes.
Stoichiometry for this reaction:
Ilmenite:
FeTiO3 + 4C ---->Fe + TiC + 3CO
Ilmenite and Rutile:
FeTiO3 + nTiO2 + (4+3n)C ---->Fe + (1+n)TiC + (3+2n)CO
Where n represents the number of TiO2 molecules
Reduction with CO
This reaction is based on a fluidized bed scheme which is similar to large scale proposals for Hydrogen Reduction. The product of CO reduction of Ilmenite is Carbon Dioxide (CO2), which is reduced to CO and oxygen. The CO is recirculated, and the oxygen stored.[2]. The presence of solar wind implanted carbon in the regolith will allow the recovery of additional carbon if recycling efficiency is sufficiently great, though a method for dealing with evolved methane (CH4, from hydrogen present in the regolith) would be required. The CO reduction of ilmenite is slower than the H2 process, but by less than an order of magnitude for any given temperature.
The reaction sequence is:
Reduction:
FeTiO3 + CO ---->Fe + TiO2 + CO2
Endothermic cracking:
2CO2 ----> 2CO + O2
Net Reaction:
2FeTiO3 + 2CO ---->2Fe + 2TiO2 + 2CO + O2
Methane Reduction
| Please note: Methane Reduction This section is a placeholder for work currently in progress. |
Li or Na Reduction
Plasma Reduction
Electrolytic Reduction
References
Related Pages
External Links
- ISRU on the Moon. by Larry Taylor http://www.lpi.usra.edu/lunar_knowledge/LTaylor.pdf
- Extraction Techniques-Oxygen. G. L. Kulcinski, February 18, 2004 http://fti.neep.wisc.edu/neep533/SPRING2004/lecture13.pdf
- Processing Lunar Soils for Oxygen and Other Materials. Knudsen & Gibson http://nss.org/settlement/nasa/spaceresvol3/plsoom1.htm
- Lunar Oxygen Production - A Maturing Technology
- The Effect of TiO2 on Synthesizing Fe-TiC Composites
- Resources of Near-Earth Space. Univ. of Arizona Press