Difference between revisions of "JSC-1"
Jarogers2001 (talk | contribs) |
Jarogers2001 (talk | contribs) |
||
| Line 237: | Line 237: | ||
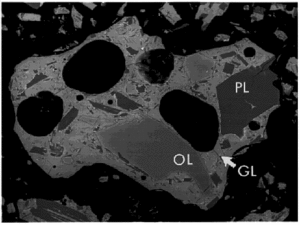
SEM back-scattered electron image. Frame width = 660 μm. Photo Credit: NASA]] | SEM back-scattered electron image. Frame width = 660 μm. Photo Credit: NASA]] | ||
--> | --> | ||
| + | |||
| + | The plagioclase crystals are needle-shaped or blocky, as large as several hundred micrometers. Pyroxene and olivine crystals are blocky to subrounded, and up to 100 µm across. Ilmenite and chromite occur as swarms of rounded crystals, each less than 10 µm in diameter. | ||
| + | |||
| + | Approximately half of the volume of a typical particle is glass of basaltic composition. Much of this glass contains plagioclase needles and oxide minerals a few micrometers in size. | ||
| + | |||
| + | ===Particle Description=== | ||
| + | Scanning electron micrographs show broken glass and mineral fragments as large as several hundred micrometers (Figure 2). Glassy particles invariably display broken vesicles with sharp edges. Mineral fragments are angular to sub-rounded, and many show the scars of impacts from the milling process. | ||
| + | |||
| + | ===Particle Size Distribution=== | ||
| + | Two particle size distribution curves for JSC-1 are presented in Figure 3. In work done at the University of Texas, Dallas (UTD curve), fifteen 250 g splits were analyzed. The samples were initially sieved dry, wetted to remove adhering fines, dried, and resieved. Finally, the weight per cent smaller than a given sieve opening was computed. | ||
| + | |||
| + | An independent analysis at the Johnson Space Center (NASA curve) followed procedures developed for lunar soil samples (McKay et al, 1974). Four 25 g splits were mixed, and a 15 g subsample was separated. This material was sieved while being wetted with freon. | ||
| + | |||
| + | The median particle sizes for these samples, defined such that 50% of the soil is larger and 50% is smaller, equal 98 µm (UTD) and 117 µm (NASA). The mean particle sizes, defined as the average of the diameters at 16, 50 and 84 wt. % smaller on the curves (Figure 3), equal 81 µm (UTD) and 105 µm (NASA). The similarity of results from the two methods provides confidence in the large-scale sample homogeneity. | ||
| + | |||
| + | ===Specific Gravity=== | ||
| + | |||
| + | The average specific gravity of JSC-1 particles is 2.9 g/cm3. This value is the ratio of particle mass to the mass of an equal volume of water measured at 4°C. Specific gravity was determined using the method of Lambe and Whitman (1969). | ||
| + | |||
| + | ===Angle of Internal Friction and Cohesion=== | ||
| + | |||
| + | The angle of internal friction for JSC-1 is approximately 45° and the cohesion of the material is approximately 1.0 kPa. These values were determined from the Mohr-Coulomb failure criterion as described by Das (1985). Samples were run in a triaxial cell and tested at confining pressures of 5, 10, and 15 psi (Turk, 1992). As shown in Figure 4, the failure envelope may be slightly nonlinear. If so, low confining pressures yield a higher angle of internal friction and lower cohesion, and vice versa. | ||
==External Links== | ==External Links== | ||
*[http://www.lpi.usra.edu/lunar/strategies/jsc_lunar_simulant.pdf NASA JSC-1 Lunar Simulant - LPI] | *[http://www.lpi.usra.edu/lunar/strategies/jsc_lunar_simulant.pdf NASA JSC-1 Lunar Simulant - LPI] | ||
Revision as of 10:57, 10 August 2008
JSC-1, a lunar soil simulant, was developed and characterized under the auspices of the NASA Johnson Space Center. This simulant was produced in large quantities to satisfy the requirements of a variety of scientific and engineering investigations. JSC-1 is derived from volcanic ash of basaltic composition, which has been ground, sized, and placed into storage. The simulant's chemical composition, mineralogy, particle size distribution, specific gravity, angle of internal friction, and cohesion have been characterized and fall within the ranges of lunar mare soil samples.
JSC-1 was produced specifically for large- and medium-scale engineering studies in support of future human activities on the Moon. Such studies include material handling, construction, excavation, and transportation. The simulant is also appropriate for research on dust control, spacesuit durability, and agriculture. JSC-1 is currently being used in studies of oxygen production and sintering. The simulant is available in large quantities to any qualified investigator.
This material complements, but does not replace, lunar simulant MLS-1, produced by the University of Minnesota (Weiblen et al, 1990). MLS-1 is derived from a high-titanium basalt hornfels which approximates the chemical composition of Apollo 11 soil The starting material is totally crystalline. As described below, JSC-1 approximates a low-titanium mare soil, and contains a high percentage of glass.
Contents
Source
JSC-1 was mined from a volcanic ash deposit located in the San Francisco volcano field near Flagstaff, AZ. This ash was erupted from vents related to Merriam Crater (35°20' N, 111°17' W). One basalt flow from a nearby vent has a K-Ar age of 0.15 ± 0.03 million years. The exposure is described as "airfall ash and lapilli, usually black, locally red, as much as several meters thick ... (which) forms broad, smooth-surfaced deposits over large areas" (Moore and Wolfe, 1987). The source quarry is within an area mapped by these authors as "slightly porphyritic basalt".
Preparation
The ash was mined from a commercial cinder quarry near the south flank of Merriam Crater. Following coarse sieving the ash was comminuted in an impact mill. This method broke down the material by means of multiple impacts with other ash particles, resulting in minimal metal contamination. The ash from several millings was allowed to partially dry in air and was then mixed. The average water content of the final mix was 2.70 ± 0.31 wt. %. The material, in 45 - 50 lb quantities, was loaded into plastic bags and the bags were heat sealed.
Characterization
Chemical Composition
The results of x-ray fluorescence (XRF) analysis are presented in Table 1. Samples were allowed to dry in air for approximately two months prior to analysis. The crushed rock was ground to pass an 80 mesh (177 μm) sieve. Oxide abundances, loss on ignition, and Fe2O3 / FeO partitioning were determined by the methods of Boyd and Mertzman (1987).
The loss on ignition (LOI) value was derived by heating samples in argon for one hour at 900°C. This value reflects the loss of volatiles, including water as well as sulfur and chlorine compounds. The analyses were performed on well-dried samples, and reflect lower water contents than those of samples from newly-opened bags.
| Table 1: Major Element Compositions | |||||
| JSC-1 (mean of 3) |
Lunar Soil 14163* | ||||
| Oxide | Conc. | Std. Dev. | Conc. | ||
| Wt % | Wt % | Wt % | |||
| SiO2 | 47.71 | 0.10 | 47.3 | ||
| TiO2 | 1.59 | 0.01 | 1.6 | ||
| Al2O3 | 15.02 | 0.04 | 17.8 | ||
| Fe2O3 | 3.44 | 0.03 | 0.0 | ||
| FeO | 7.35 | 0.05 | 10.5 | ||
| MgO | 9.01 | 0.09 | 9.6 | ||
| CaO | 10.42 | 0.03 | 11.4 | ||
| Na2O | 2.70 | 0.03 | 0.7 | ||
| K2O | 0.82 | 0.02 | 0.6 | ||
| MnO | 0.18 | 0.00 | 0.1 | ||
| Cr2O3 | 0.04 | 0.00 | 0.2 | ||
| P2O5 | 0.66 | 0.01 | ... | ||
| LOI | 0.71 | 0.05 | ... | ||
| Total | 99.65 | 99.8 | |||
| LOI = Loss on ignition * Papike et al (1982) | |||||
The results of x-ray fluorescence (XRF) analysis are presented in Table 1. Samples were allowed to dry in air for approximately two months prior to analysis. The crushed rock was ground to pass an 80 mesh (177 μm) sieve. Oxide abundances, loss on ignition, and Fe2O3 / FeO partitioning were determined by the methods of Boyd and Mertzman (1987).
| Table 2. Trace Element Concentrations in JSC-1 | |||||
| Element | Conc. (ppm) |
Std. Dev. (ppm) |
Element | Conc. (ppm) |
Std. Dev. (ppm) |
| Sc | 29.2 | 0.5 | Yb | 1.99 | 0.04 |
| Co | 47.7 | 1.6 | Zr | 125 | 3 |
| Ni | 137 | 18 | Hf | 3.55 | 0.08 |
| Rb | 12.3 | 1.5 | Ta | 1.96 | 0.04 |
| Cs | 0.339 | 0.012 | U | 1.51 | 0.08 |
| Sr | 860 | 36 | Th | 5.65 | 0.07 |
| Ba | 822 | 13 | As | 18.7 | 8.9 |
| La | 48.2 | 0.9 | Se | < 0.5 | 0.0 |
| Ce | 94.6 | 1.7 | Sb | 0.564 | 0.569 |
| Nd | 42 | 2 | W | 36.1 | 2.6 |
| Sm | 7.44 | 0.13 | Au (ppb) | 40.7 | 29.4 |
| Eu | 2.18 | 0.04 | Br | 0.85 | 0.07 |
| Tb | 0.825 | 0.012 | Lu | 0.293 | 0.007 |
The loss on ignition (LOI) value was derived by heating samples in argon for one hour at 900°C. This value reflects the loss of volatiles, including water as well as sulfur and chlorine compounds. The analyses were performed on well-dried samples, and reflect lower water contents than those of samples from newly-opened bags.
The trace element concentrations in JSC-1 are listed in Table 2. These data were averaged from instrumental neutron activation analyses (INAA) performed on three samples. The samples, loaded into pure silica tubes, were irradiated the University of Missouri Research Reactor Facility for 20 hours each at a flux of 7.6 x 1013 n/cm2-sec. Counting and data reduction were performed at the Johnson Space Center.
Mineralogy
The major mineral species in JSC-1 samples were identified by x-ray diffraction (XRD), optical microscopy, and scanning electron microscopy (SEM). Qualitative elemental abundances were determined for some SEM samples by energy-dispersive x-ray spectrometry (EDS). The major crystalline phases are plagioclase, pyroxene and olivine. Minor minerals include the oxides ilmenite and chromite, plus traces of clay. The glass and minerals in a typical grain are shown in Figure 1.
The plagioclase crystals are needle-shaped or blocky, as large as several hundred micrometers. Pyroxene and olivine crystals are blocky to subrounded, and up to 100 µm across. Ilmenite and chromite occur as swarms of rounded crystals, each less than 10 µm in diameter.
Approximately half of the volume of a typical particle is glass of basaltic composition. Much of this glass contains plagioclase needles and oxide minerals a few micrometers in size.
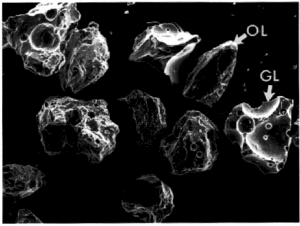
Particle Description
Scanning electron micrographs show broken glass and mineral fragments as large as several hundred micrometers (Figure 2). Glassy particles invariably display broken vesicles with sharp edges. Mineral fragments are angular to sub-rounded, and many show the scars of impacts from the milling process.
Particle Size Distribution
Two particle size distribution curves for JSC-1 are presented in Figure 3. In work done at the University of Texas, Dallas (UTD curve), fifteen 250 g splits were analyzed. The samples were initially sieved dry, wetted to remove adhering fines, dried, and resieved. Finally, the weight per cent smaller than a given sieve opening was computed.
An independent analysis at the Johnson Space Center (NASA curve) followed procedures developed for lunar soil samples (McKay et al, 1974). Four 25 g splits were mixed, and a 15 g subsample was separated. This material was sieved while being wetted with freon.
The median particle sizes for these samples, defined such that 50% of the soil is larger and 50% is smaller, equal 98 µm (UTD) and 117 µm (NASA). The mean particle sizes, defined as the average of the diameters at 16, 50 and 84 wt. % smaller on the curves (Figure 3), equal 81 µm (UTD) and 105 µm (NASA). The similarity of results from the two methods provides confidence in the large-scale sample homogeneity.
Specific Gravity
The average specific gravity of JSC-1 particles is 2.9 g/cm3. This value is the ratio of particle mass to the mass of an equal volume of water measured at 4°C. Specific gravity was determined using the method of Lambe and Whitman (1969).
Angle of Internal Friction and Cohesion
The angle of internal friction for JSC-1 is approximately 45° and the cohesion of the material is approximately 1.0 kPa. These values were determined from the Mohr-Coulomb failure criterion as described by Das (1985). Samples were run in a triaxial cell and tested at confining pressures of 5, 10, and 15 psi (Turk, 1992). As shown in Figure 4, the failure envelope may be slightly nonlinear. If so, low confining pressures yield a higher angle of internal friction and lower cohesion, and vice versa.