Difference between revisions of "JSC-1"
(adding category) |
|||
| (5 intermediate revisions by 4 users not shown) | |||
| Line 12: | Line 12: | ||
JSC-1 was mined from a volcanic ash deposit located in the San Francisco volcano field near Flagstaff, AZ. This ash was erupted from vents related to Merriam Crater (35°20' N, 111°17' W). One basalt flow from a nearby vent has a K-Ar age of 0.15 ± 0.03 million years. The exposure is described as "airfall ash and lapilli, usually black, locally red, as much as several meters thick ... (which) forms broad, smooth-surfaced deposits over large areas<ref name="moore">Moore R.B. and Wolfe E.W. (1987) Geologic Map of the East Part of the San Francisco Volcanic Field, North-Central Arizona, Map MF-1960, U.S. Geological Survey, Washington, D.C.</ref>". The source quarry is within an area mapped by these authors as "slightly porphyritic basalt". | JSC-1 was mined from a volcanic ash deposit located in the San Francisco volcano field near Flagstaff, AZ. This ash was erupted from vents related to Merriam Crater (35°20' N, 111°17' W). One basalt flow from a nearby vent has a K-Ar age of 0.15 ± 0.03 million years. The exposure is described as "airfall ash and lapilli, usually black, locally red, as much as several meters thick ... (which) forms broad, smooth-surfaced deposits over large areas<ref name="moore">Moore R.B. and Wolfe E.W. (1987) Geologic Map of the East Part of the San Francisco Volcanic Field, North-Central Arizona, Map MF-1960, U.S. Geological Survey, Washington, D.C.</ref>". The source quarry is within an area mapped by these authors as "slightly porphyritic basalt". | ||
| − | + | ==Preparation== | |
| + | The ash was mined from a commercial cinder quarry near the south flank of Merriam Crater. Following coarse sieving the ash was comminuted in an impact mill. This method broke down the material by means of multiple impacts with other ash particles, resulting in minimal metal contamination. The ash from several millings was allowed to partially dry in air and was then mixed. The average water content of the final mix was 2.70 ± 0.31 wt. %. The material, in 45 - 50 lb quantities, was loaded into plastic bags and the bags were heat sealed. | ||
==Characterization== | ==Characterization== | ||
| Line 270: | Line 271: | ||
SEM back-scattered electron image. Frame width = 660 μm. Photo Credit: NASA]] | SEM back-scattered electron image. Frame width = 660 μm. Photo Credit: NASA]] | ||
| − | + | ===Mineralogy=== | |
| + | |||
| + | The minerals found in JSC-1, plagioclase, pyroxene, olivine, ilmenite, and chromite, are also characteristic of many lunar basalts and mare soils (Figure 5). The compositional ranges of these lunar minerals generally overlap the ranges of their terrestrial counterparts. | ||
| + | Lunar soil also contains a significant component of glass produced by micrometeorite impact. This "agglutinitic" glass was apparently formed under highly reducing conditions, as it invariably contains submicrometer particles of native iron metal. The volcanic glass component of JSC-1 contains more micrometer-scale plagioclase and metal oxide crystals than lunar impact glass, but the JSC-1 glass contains no iron metal. | ||
| + | |||
| + | ===Particle Description=== | ||
| + | |||
| + | Lunar soil is a complex mixture of rock and mineral grains shattered by impact, along with impact-derived glass. This agglutinitic glass bonds the rock and mineral fragments into submillimeter particles which are characteristic of most lunar soils (Figure 5). Such particles constitute over half of the volume of many soil samples. The micrometer-scale textures of lunar agglutinates are extremely complex, and are not precisely matched by any terrestrial analog. | ||
===Particle Size Distribution=== | ===Particle Size Distribution=== | ||
| Line 327: | Line 335: | ||
The angle of internal friction for lunar soil samples ranges from 25° - 50° <ref name="carrier">Carrier W.D. III, Olhoeft G.R., and Mendell W. (1991) Physical properties of the lunar surface in Lunar Sourcebook (G.H. Heiken, D.T. Vaniman, and B.M. French, Eds.), Cambridge University Press, Cambridge, 736 pp.</ref>. The cohesion values for these samples ranges from 0.26 - 1.8 kPa <ref name="carrier"/>. JSC-1, with values of 45° and 1.0 kPa, respectively, is a good mechanical analog to lunar soil. | The angle of internal friction for lunar soil samples ranges from 25° - 50° <ref name="carrier">Carrier W.D. III, Olhoeft G.R., and Mendell W. (1991) Physical properties of the lunar surface in Lunar Sourcebook (G.H. Heiken, D.T. Vaniman, and B.M. French, Eds.), Cambridge University Press, Cambridge, 736 pp.</ref>. The cohesion values for these samples ranges from 0.26 - 1.8 kPa <ref name="carrier"/>. JSC-1, with values of 45° and 1.0 kPa, respectively, is a good mechanical analog to lunar soil. | ||
| − | + | ==External Links== | |
| + | |||
| + | *[http://ares.jsc.nasa.gov/HumanExplore/Exploration/EXLibrary/DOCS/EIC050.HTML JSC-1 Lunar Simulant - NASA] - The original version of this article which was funded by NASA and written by [[David S. McKay]], [[James L. Carter]], [[Walter W. Boles]], [[Carlton C. Allen]], and [[Judith H. Allton]]. | ||
==References== | ==References== | ||
| Line 339: | Line 349: | ||
--> | --> | ||
| + | |||
| + | [[Category:Selenology]] | ||
Latest revision as of 16:25, 17 January 2012
JSC-1, a lunar soil simulant, was developed and characterized under the auspices of the NASA Johnson Space Center. This simulant was produced in large quantities to satisfy the requirements of a variety of scientific and engineering investigations. JSC-1 is derived from volcanic ash of basaltic composition, which has been ground, sized, and placed into storage. The simulant's chemical composition, mineralogy, particle size distribution, specific gravity, angle of internal friction, and cohesion have been characterized and fall within the ranges of lunar mare soil samples.
JSC-1 was produced specifically for large- and medium-scale engineering studies in support of future human activities on the Moon. Such studies include material handling, construction, excavation, and transportation. The simulant is also appropriate for research on dust control, spacesuit durability, and agriculture. JSC-1 is currently being used in studies of oxygen production and sintering. The simulant is available in large quantities to any qualified investigator.
This material complements, but does not replace, lunar simulant MLS-1, produced by the University of Minnesota[1]. MLS-1 is derived from a high-titanium basalt hornfels which approximates the chemical composition of Apollo 11 soil The starting material is totally crystalline. As described below, JSC-1 approximates a low-titanium mare soil, and contains a high percentage of glass.
Contents
Source
JSC-1 was mined from a volcanic ash deposit located in the San Francisco volcano field near Flagstaff, AZ. This ash was erupted from vents related to Merriam Crater (35°20' N, 111°17' W). One basalt flow from a nearby vent has a K-Ar age of 0.15 ± 0.03 million years. The exposure is described as "airfall ash and lapilli, usually black, locally red, as much as several meters thick ... (which) forms broad, smooth-surfaced deposits over large areas[2]". The source quarry is within an area mapped by these authors as "slightly porphyritic basalt".
Preparation
The ash was mined from a commercial cinder quarry near the south flank of Merriam Crater. Following coarse sieving the ash was comminuted in an impact mill. This method broke down the material by means of multiple impacts with other ash particles, resulting in minimal metal contamination. The ash from several millings was allowed to partially dry in air and was then mixed. The average water content of the final mix was 2.70 ± 0.31 wt. %. The material, in 45 - 50 lb quantities, was loaded into plastic bags and the bags were heat sealed.
Characterization
Chemical Composition
The results of x-ray fluorescence (XRF) analysis are presented in Table 1. Samples were allowed to dry in air for approximately two months prior to analysis. The crushed rock was ground to pass an 80 mesh (177 μm) sieve. Oxide abundances, loss on ignition, and Fe2O3 / FeO partitioning were determined by the methods of Boyd and Mertzman (1987)[3].
The loss on ignition (LOI) value was derived by heating samples in argon for one hour at 900°C. This value reflects the loss of volatiles, including water as well as sulfur and chlorine compounds. The analyses were performed on well-dried samples, and reflect lower water contents than those of samples from newly-opened bags.
| Table 1: Major Element Compositions | |||||
| JSC-1 (mean of 3) |
Lunar Soil 14163* | ||||
| Oxide | Conc. | Std. Dev. | Conc. | ||
| Wt % | Wt % | Wt % | |||
| SiO2 | 47.71 | 0.10 | 47.3 | ||
| TiO2 | 1.59 | 0.01 | 1.6 | ||
| Al2O3 | 15.02 | 0.04 | 17.8 | ||
| Fe2O3 | 3.44 | 0.03 | 0.0 | ||
| FeO | 7.35 | 0.05 | 10.5 | ||
| MgO | 9.01 | 0.09 | 9.6 | ||
| CaO | 10.42 | 0.03 | 11.4 | ||
| Na2O | 2.70 | 0.03 | 0.7 | ||
| K2O | 0.82 | 0.02 | 0.6 | ||
| MnO | 0.18 | 0.00 | 0.1 | ||
| Cr2O3 | 0.04 | 0.00 | 0.2 | ||
| P2O5 | 0.66 | 0.01 | ... | ||
| LOI | 0.71 | 0.05 | ... | ||
| Total | 99.65 | 99.8 | |||
| LOI = Loss on ignition * Papike et al (1982)[4] | |||||
The results of x-ray fluorescence (XRF) analysis are presented in Table 1. Samples were allowed to dry in air for approximately two months prior to analysis. The crushed rock was ground to pass an 80 mesh (177 μm) sieve. Oxide abundances, loss on ignition, and Fe2O3 / FeO partitioning were determined by the methods of Boyd and Mertzman (1987)[3].
| Table 2. Trace Element Concentrations in JSC-1 | |||||
| Element | Conc. (ppm) |
Std. Dev. (ppm) |
Element | Conc. (ppm) |
Std. Dev. (ppm) |
| Sc | 29.2 | 0.5 | Yb | 1.99 | 0.04 |
| Co | 47.7 | 1.6 | Zr | 125 | 3 |
| Ni | 137 | 18 | Hf | 3.55 | 0.08 |
| Rb | 12.3 | 1.5 | Ta | 1.96 | 0.04 |
| Cs | 0.339 | 0.012 | U | 1.51 | 0.08 |
| Sr | 860 | 36 | Th | 5.65 | 0.07 |
| Ba | 822 | 13 | As | 18.7 | 8.9 |
| La | 48.2 | 0.9 | Se | < 0.5 | 0.0 |
| Ce | 94.6 | 1.7 | Sb | 0.564 | 0.569 |
| Nd | 42 | 2 | W | 36.1 | 2.6 |
| Sm | 7.44 | 0.13 | Au (ppb) | 40.7 | 29.4 |
| Eu | 2.18 | 0.04 | Br | 0.85 | 0.07 |
| Tb | 0.825 | 0.012 | Lu | 0.293 | 0.007 |
The loss on ignition (LOI) value was derived by heating samples in argon for one hour at 900°C. This value reflects the loss of volatiles, including water as well as sulfur and chlorine compounds. The analyses were performed on well-dried samples, and reflect lower water contents than those of samples from newly-opened bags.
The trace element concentrations in JSC-1 are listed in Table 2. These data were averaged from instrumental neutron activation analyses (INAA) performed on three samples. The samples, loaded into pure silica tubes, were irradiated the University of Missouri Research Reactor Facility for 20 hours each at a flux of 7.6 x 1013 n/cm2-sec. Counting and data reduction were performed at the Johnson Space Center.
Mineralogy
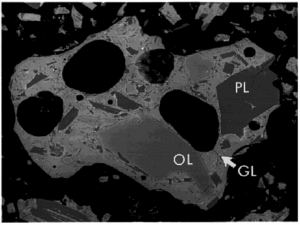
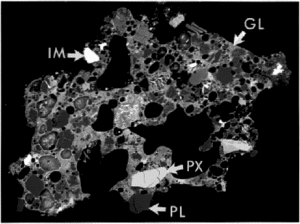
The major mineral species in JSC-1 samples were identified by x-ray diffraction (XRD), optical microscopy, and scanning electron microscopy (SEM). Qualitative elemental abundances were determined for some SEM samples by energy-dispersive x-ray spectrometry (EDS). The major crystalline phases are plagioclase, pyroxene and olivine. Minor minerals include the oxides ilmenite and chromite, plus traces of clay. The glass and minerals in a typical grain are shown in Figure 1.
The plagioclase crystals are needle-shaped or blocky, as large as several hundred micrometers. Pyroxene and olivine crystals are blocky to subrounded, and up to 100 µm across. Ilmenite and chromite occur as swarms of rounded crystals, each less than 10 µm in diameter.
Approximately half of the volume of a typical particle is glass of basaltic composition. Much of this glass contains plagioclase needles and oxide minerals a few micrometers in size.
Particle Description
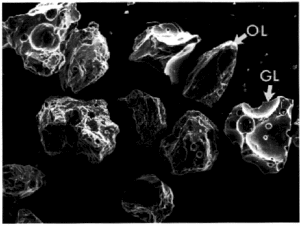
Scanning electron micrographs show broken glass and mineral fragments as large as several hundred micrometers (Figure 2). Glassy particles invariably display broken vesicles with sharp edges. Mineral fragments are angular to sub-rounded, and many show the scars of impacts from the milling process.
Particle Size Distribution
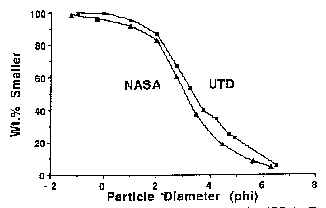
Two particle size distribution curves for JSC-1 are presented in Figure 3. In work done at the University of Texas, Dallas (UTD curve), fifteen 250 g splits were analyzed. The samples were initially sieved dry, wetted to remove adhering fines, dried, and resieved. Finally, the weight per cent smaller than a given sieve opening was computed.
An independent analysis at the Johnson Space Center (NASA curve) followed procedures developed for lunar soil samples[5]. Four 25 g splits were mixed, and a 15 g subsample was separated. This material was sieved while being wetted with freon.
The median particle sizes for these samples, defined such that 50% of the soil is larger and 50% is smaller, equal 98 µm (UTD) and 117 µm (NASA). The mean particle sizes, defined as the average of the diameters at 16, 50 and 84 wt. % smaller on the curves (Figure 3), equal 81 µm (UTD) and 105 µm (NASA). The similarity of results from the two methods provides confidence in the large-scale sample homogeneity.
Specific Gravity
The average specific gravity of JSC-1 particles is 2.9 g/cm3. This value is the ratio of particle mass to the mass of an equal volume of water measured at 4°C. Specific gravity was determined using the method of Lambe and Whitman (1969)[6].
Angle of Internal Friction and Cohesion
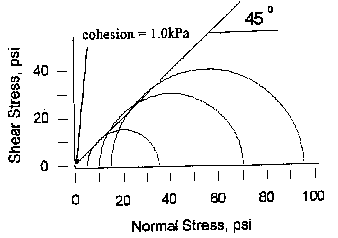
The angle of internal friction for JSC-1 is approximately 45° and the cohesion of the material is approximately 1.0 kPa. These values were determined from the Mohr-Coulomb failure criterion as described by Das (1985)[7]. Samples were run in a triaxial cell and tested at confining pressures of 5, 10, and 15 psi[8]. As shown in Figure 4, the failure envelope may be slightly nonlinear. If so, low confining pressures yield a higher angle of internal friction and lower cohesion, and vice versa.
Comparison to Lunar Soils
Chemical Composition
JSC-1 is a basaltic ash with a composition typical of many terrestrial basalts. Lunar basalts, and the mare soils derived from them, are generally similar to JSC-1 in major element composition. Lunar samples, however, contain no water and have low abundances of volatile oxides such as Na2O. In addition, lunar rocks were formed in highly reducing environments and contain iron only as Fe2+ and Fe0. These differences are illustrated in Table 1, which compares the composition of JSC-1 to that of Apollo 14 soil 14163[4].
Mineralogy
The minerals found in JSC-1, plagioclase, pyroxene, olivine, ilmenite, and chromite, are also characteristic of many lunar basalts and mare soils (Figure 5). The compositional ranges of these lunar minerals generally overlap the ranges of their terrestrial counterparts. Lunar soil also contains a significant component of glass produced by micrometeorite impact. This "agglutinitic" glass was apparently formed under highly reducing conditions, as it invariably contains submicrometer particles of native iron metal. The volcanic glass component of JSC-1 contains more micrometer-scale plagioclase and metal oxide crystals than lunar impact glass, but the JSC-1 glass contains no iron metal.
Particle Description
Lunar soil is a complex mixture of rock and mineral grains shattered by impact, along with impact-derived glass. This agglutinitic glass bonds the rock and mineral fragments into submillimeter particles which are characteristic of most lunar soils (Figure 5). Such particles constitute over half of the volume of many soil samples. The micrometer-scale textures of lunar agglutinates are extremely complex, and are not precisely matched by any terrestrial analog.
Particle Size Distribution
| Table 3. Mean and Median Particle Sizes | |
| Sample | Size Range (µm) |
| JSC-1 (median - UTD) | 98 |
| JSC-1 (median - NASA) | 117 |
| JSC-1 (mean - UTD) | 81 |
| JSC-1 (mean - NASA) | 105 |
| Apollo 11 (median)* | 48 - 105 |
| Apollo 12 (median)* | 42 - 94 |
| Apollo 14 (median)* | 75 - 802 |
| Apollo 15 (median)* | 51 - 108 |
| Apollo 16 (mean)* | 101 - 268 |
| Apollo 17 (mean)* | 42 - 166 |
| *(McKay et al, 1991; Table 7.8)[9] | |
JSC-1 is similar in particle size distribution to a typical submature lunar soil, such as Apollo 15 soil 15530 with a median particle size of 102 µm. Mean and median particle size ranges from Apollo soil samples are compared in Table 3.
JSC-1 grains are better sorted, i.e., have a narrower particle size distribution, than most lunar soils. However, several samples, such as Apollo 14 soil 14230 and Apollo 17 core 74002, have comparably high degrees of sorting.
An extensive compilation of 143 lunar soil particle size distributions has recently been published by Graf (1993)[10]. Investigators desiring to compare JSC-1 to a specific soil sample should consult this volume.
Specific Gravity
The specific gravity values for lunar soil samples range from 2.9 - 3.5 g/cm3 [11]. The JSC-1 value, 2.9 g/cm3, falls on the low end of this range.
Angle of Internal Friction and Cohesion
The angle of internal friction for lunar soil samples ranges from 25° - 50° [11]. The cohesion values for these samples ranges from 0.26 - 1.8 kPa [11]. JSC-1, with values of 45° and 1.0 kPa, respectively, is a good mechanical analog to lunar soil.
External Links
- JSC-1 Lunar Simulant - NASA - The original version of this article which was funded by NASA and written by David S. McKay, James L. Carter, Walter W. Boles, Carlton C. Allen, and Judith H. Allton.
References
- ↑ Weiblen P.W., Murawa M.J., and Reid K.J. (1990) Preparation of simulants for lunar surface materials. Engineering, Construction and Operations in Space II, American Society of Civil Engineers, New York, pp. 428-435.
- ↑ Moore R.B. and Wolfe E.W. (1987) Geologic Map of the East Part of the San Francisco Volcanic Field, North-Central Arizona, Map MF-1960, U.S. Geological Survey, Washington, D.C.
- ↑ 3.0 3.1 Boyd F.R. and Mertzman S.A. (1987) Composition and structure of the Kaapvaal lithosphere, Southern Africa, in Magmatic Processes (B.O. Mysen, Ed.), Geochem. Soc. Spec. Publ. 1, pp. 13-24.
- ↑ 4.0 4.1 Papike J.J., Simon S.B., and Laul J.C. (1982) The lunar regolith: chemistry, mineralogy and petrology, Rev. Geophys. Space Phys., 20, pp. 761-826.
- ↑ McKay D.S., Fruland, R.M., and Heiken G.H. (1974) Grain size and the evolution of lunar soils. Proceedings of the Fifth Lunar Science Conference, pp. 829-841.
- ↑ Lambe T.W. and Whitman R.V. (1969) Soil Mechanics, John Wiley and Sons, Inc., New York.
- ↑ Das B.M. (1985) Principles of Geotechnical Engineering, PWS-Kent, Inc., Boston.
- ↑ Turk, B.L., Jr. (1992) Laboratory Soil Testing for Engineers, Dept. of Civil Engineering, Texas A&M University, College Station, TX.
- ↑ McKay D.S., Heiken G., Basu A., Blanford G., Simon S., Reedy R., French B.M., and Papike, J. (1991) The lunar regolith, in Lunar Sourcebook (G.H. Heiken, D.T. Vaniman, and B.M. French, Eds.), Cambridge University Press, Cambridge, 736 pp.
- ↑ Graf J.C. (1993) Lunar soils grain size catalog, NASA RP 1265, National Aeronautics and Space Administration, Washington, DC, 466 pp
- ↑ 11.0 11.1 11.2 Carrier W.D. III, Olhoeft G.R., and Mendell W. (1991) Physical properties of the lunar surface in Lunar Sourcebook (G.H. Heiken, D.T. Vaniman, and B.M. French, Eds.), Cambridge University Press, Cambridge, 736 pp.